Содержание
- 2. Acknowledgements Prof. GuoLiang Chen; Prof. Hywel A Davies; Prof. Peter K Liaw; Prof. George Smith; Prof.
- 3. Outlines I. Background & Motivations II. Results & Discussions III. Summaries
- 4. (1) Conventional alloys I. Background & Motivations Steel, A=Fe, B=Carbon, δB Cast Iron, A=Fe, B=Carbon, δB
- 5. (2) High Entropy Alloys HEAs=A+B+C+D+E; 50% 15% AlCoCrFeNi=HEA , Zhou, APL, 2007 CoCrCuFeNi=HEA, Yeh, MMTA, 2004;
- 6. Solid solution has higher entropy than the mechanical mixture does. 1.2 Thermodynamically For the regular solution:
- 7. Gibbs Free Energy ΔGmix =ΔHmix-TΔSmix
- 8. 1.3 Properties and Applications High Strength; Zhou, APL, 2007; High wear resistance; Lin, Surface Coating technology,
- 9. 1 Coatings, Barriers, etc. Diffusion barriers for Cu interconnections; Tsai, APL, 2008 2 Structural Materials 3
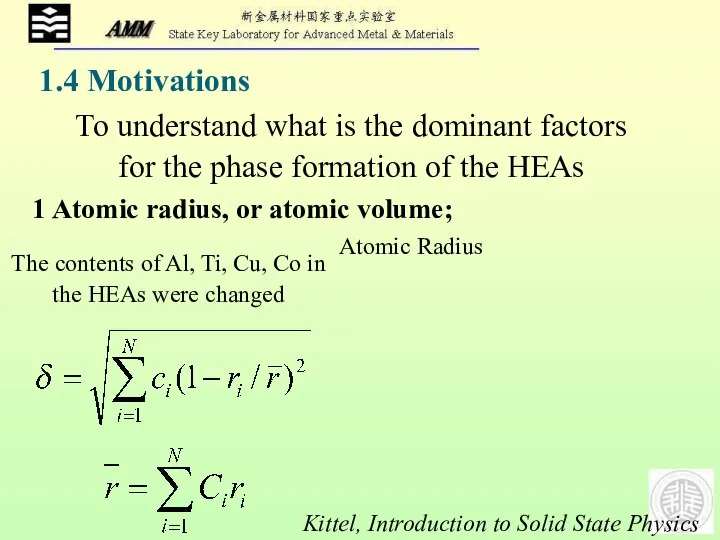
- 10. To understand what is the dominant factors for the phase formation of the HEAs 1 Atomic
- 11. 2 Enthalpy of Mixing; 3 Entropy of Mixing
- 12. 4 Cooling Rate 5 Tensile and compressive properties Critical cooling rate? Like the BMG? Tensile elongation=0?
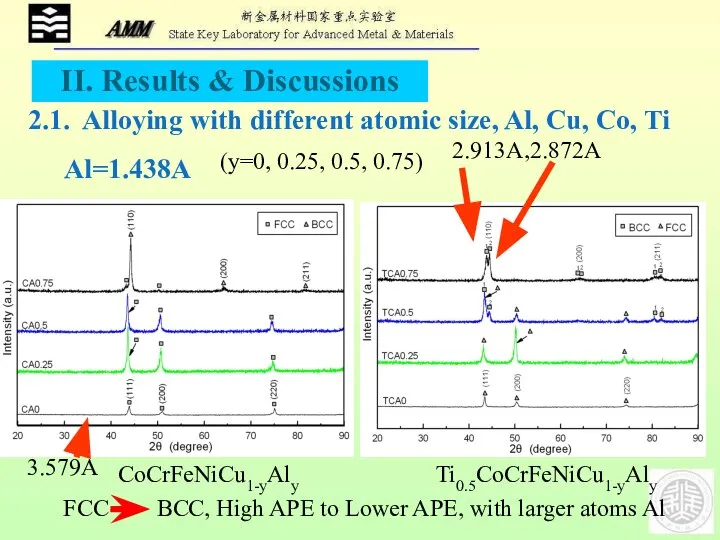
- 13. CoCrFeNiCu1-yAly FCC BCC, High APE to Lower APE, with larger atoms Al 2.1. Alloying with different
- 14. Cu=1.278A CoCrFeNiAlCuy ( y=0, 0.25, 0.5) Ti0.5CoCrFeNiAlCuy No PHASE TRANSITION
- 15. Co=1.251A The smaller BCC transit to FCC firstly after adding Co Biger BCC1phase:2.913A; Smaller BCC2phase:2.872A
- 16. [Al1Co1Cr1Fe1Ni1]Tix alloys BCC+Ti BCC+BCC Ti=1.448A
- 17. After adding Ti, Laves phase forms
- 18. Zhou, APL, 2008 The transition is mainly lattice distortion induced and APE related
- 19. A schematic showing the additional effects
- 20. Zhang, AEM, 2008 2.2. Considering of the enthalpy of mixing ΔHmix Mg based BMG Zr based
- 21. 2.3. Considering of the entropy of mixing ΔSmix High Entropy is not good for the formation
- 22. 2.4 Cooling Rate AlCoCrFeNi
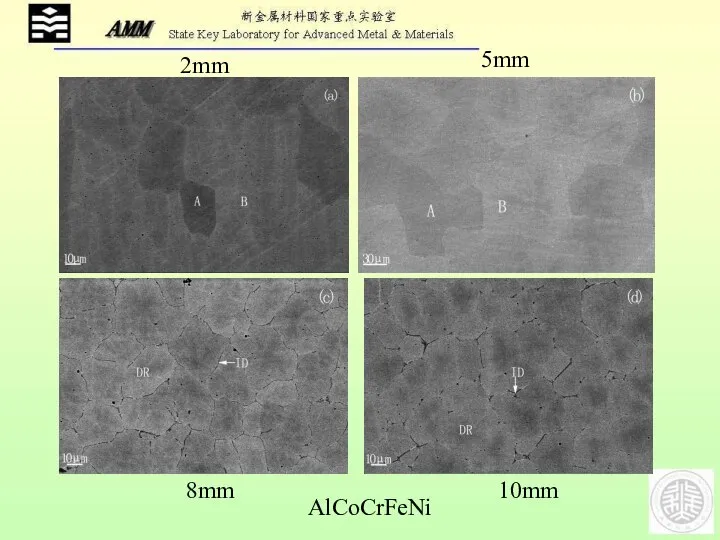
- 23. AlCoCrFeNi 2mm 5mm 8mm 10mm
- 24. AlCoCrFeNi
- 25. 2.5 Tensile and Compressive properties XRD pattern for the CoCrCuFeNiAl0.5 alloy.
- 26. Table Room temperature mechanical test results for the CoCrCuFeNiAl0.5 alloy εP: plastic strain; ε0.2 : yield
- 27. III. Summaries 1 Atomic size mismatch is the dominant factor for the phase formation of the
- 29. Скачать презентацию











![[Al1Co1Cr1Fe1Ni1]Tix alloys BCC+Ti BCC+BCC Ti=1.448A](/_ipx/f_webp&q_80&fit_contain&s_1440x1080/imagesDir/jpg/1492876/slide-15.jpg)










 Реактивтермен жұмыс істеу техникасы және тұздар, қышқылдар, ерітінділер
Реактивтермен жұмыс істеу техникасы және тұздар, қышқылдар, ерітінділер Кислотно-основное равновесие в процессах жизнедеятельности. Ионное произведение воды. Водородный показатель
Кислотно-основное равновесие в процессах жизнедеятельности. Ионное произведение воды. Водородный показатель Жири. Склад жирів, їх утворення. Жири у природі. Біологічна роль жирів.
Жири. Склад жирів, їх утворення. Жири у природі. Біологічна роль жирів.  Свойства растворов. Протолитическая теория кислот и оснований Бренстеда-Лоури
Свойства растворов. Протолитическая теория кислот и оснований Бренстеда-Лоури «Введение в органическую химию» Технология урока. Учитель химии высшей категории МКОУ СОШ с УИОП пгт. Нагорск Антышева Нина Але
«Введение в органическую химию» Технология урока. Учитель химии высшей категории МКОУ СОШ с УИОП пгт. Нагорск Антышева Нина Але Липиды. Определение. Классификация
Липиды. Определение. Классификация Химические волокна
Химические волокна Варианты заданий к расчету однократной экстракции
Варианты заданий к расчету однократной экстракции «Тайна Воды», или немного о свойствах воды
«Тайна Воды», или немного о свойствах воды Техника безопасности на уроках химии_
Техника безопасности на уроках химии_ Реакции замещения
Реакции замещения Классификация минералов
Классификация минералов Химия - әлемді тану құралы. (11 класс)
Химия - әлемді тану құралы. (11 класс) Как осуществить цепочку превращений на основании положений теории электролитической диссоциации?
Как осуществить цепочку превращений на основании положений теории электролитической диссоциации? Основные способы получения металлов
Основные способы получения металлов Цинк (Zn). Свойства
Цинк (Zn). Свойства Каталитический крекинг
Каталитический крекинг Петрология. Классификации магматических горных пород
Петрология. Классификации магматических горных пород Реакции замещения
Реакции замещения Сульфат меди
Сульфат меди Спирты: классификация, изомерия, номенклатура Подготовила учитель химии Несмеянова М.В. МОУ «Сытьковская СОШ»Рузский район М
Спирты: классификация, изомерия, номенклатура Подготовила учитель химии Несмеянова М.В. МОУ «Сытьковская СОШ»Рузский район М The Krebs cycle
The Krebs cycle Углеводороды нефти. Алканы. Парафины Циклоалканы. Нафтены Ароматические углеводороды. Арены
Углеводороды нефти. Алканы. Парафины Циклоалканы. Нафтены Ароматические углеводороды. Арены Методы снижения уровня учебного стресса у обучающихся 9-х классов на уроках химии
Методы снижения уровня учебного стресса у обучающихся 9-х классов на уроках химии Липиды и низкомолекулярные регуляторы
Липиды и низкомолекулярные регуляторы Электролиз. Электролиз расплавов и растворов
Электролиз. Электролиз расплавов и растворов Углеводы. Общие представления об углеводах
Углеводы. Общие представления об углеводах Сульфатна кислота і сульфати
Сульфатна кислота і сульфати